
You have a vaccine and you want an innovative device to inject it?
Our disruptive technology is easy to use and safe. We offer strong filling capacities at a competitive cost!
.gif)
Euroject® advantages
- Open system, free of royalties
- Adaptable to any type of needle
- Different routes of administration for injection
- Dedicated BSL2* building in our Amiens facility
- Very large capacity: up to 1 billion doses per year
- Competitive pricing
- No risk of shortage in components and capacities
- Opportunity for new markets (LMIC*)
*Biosafety level *Low middle income countries
.png?width=1050&height=600&name=Rose%20et%20Blanc%20Minimaliste%20Maquillage%20Artiste%20Carte%20de%20visite%20(4).png)
Contact us now!
And access our Blow-Fill-Seal technical data sheets and the summary of the HCP's practice study.
Fill in our contact form above to access our Data Sheets!
- Temperature management
- Extractables & leachables
- Usability testing
- Cost-effectiveness

INJECTION DEVICE USABILITY TESTING
Qualitative study performed in the UK on April 20th. 2023 among healthcare professionals (HCPs: 6 physicians, 2 nurses), with injection experience in low income countries.
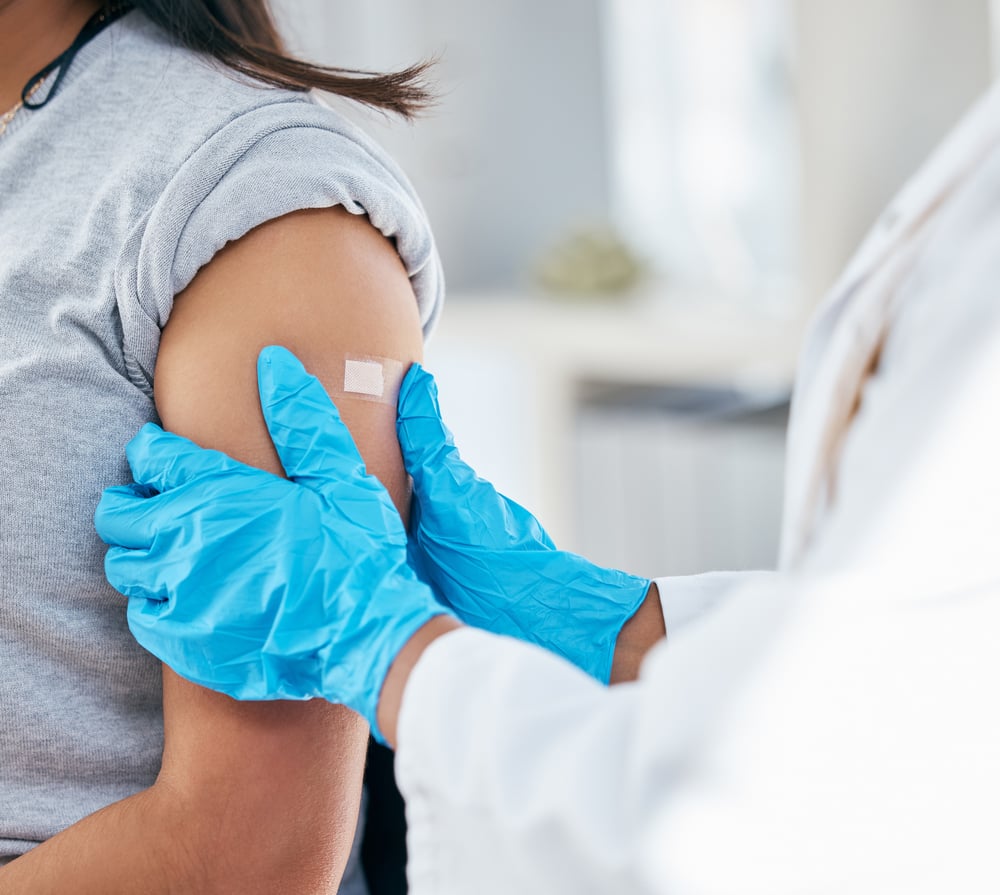
Euroject® could be the game changer for HCPs practice. Discover why!
Key information:
• Device adapted to low income countries
• Overall interest for this new injection device
• Space saving and suitable for a busy clinic environment
• Identified as a potential low cost / cost effective device
• Plastic vials sturdier (less breakable) than glass vial
• No higher cross contamination foreseen and even lower than with a manual drawing up of an injection from a vial
Blow-Fill-Seal technology
Blow-Fill-Seal (BFS) is an Advanced Aseptic process for which temperature management is essential. It performs the primary packaging for a wide product variety: nebulized inhalation drugs, ophthalmic, OTC products and drugs, and injectable products.
- Plastic is extruded at 170 – 220°C depending on the resin being used. The cooling process starts as soon as the plastic parison leaves the extruder head.
- The temperature controlled metal copper alloy molds continue the cooling process as they close and form the primary container.
.png)
Euroject® project video presentation
Vaccine manufacturing sites
Laboratoire Unither Amiens
Espace Industriel Nord
151 Rue André Durouchez
CS 28028
80084 Amiens Cedex 2
France
Tél : + 33 (0)3 22 54 73 00
Laboratoire Unither Coutances
ZI de la Guérie
50200 Coutances
France
Tél : + 33 (0)2 33 76 70 00

In the press
- International:
- Pharmanetwork: UNITHER invests 68 million euros in the innovative project: Euroject
- Pharmanetwork: Injectable Innovations
France: - Courrier Picard: Euroject® : le projet d’unité de fabrication de vaccins injectables / Unither Pharmaceuticals lance un chantier d’extension de son site de la zone industrielle Nord à Amiens
- Industrie Pharma / Usine nouvelle : CDMO : À Amiens, Unither pose la première pierre de son projet de vaccins au format unidose (usinenouvelle.com)
- France Bleu: A Amiens, Unither va confectionner des vaccins injectables contre le coronavirus
- Le Bonhomme Picard : CoviD : doses perdues, lenteur des vaccins, Unither trouve la parade (lebonhommepicard.fr)
- Amiens Métropole : Unither voit très grand - Amiens Métropole
About Unither Pharmaceuticals
Unither Pharmaceuticals is a world leading CDMO that specializes in the development and manufacturing of liquid single dose forms (mainly eye drops, saline solutions, and asthma medications in BFS unit doses and OTC and Rx formulations in liquid stick packs for pharmaceutical companies and generic manufacturers Unither Pharmaceuticals employs around 2 000 people across 8 manufacturing facilities in France, the United States, Brazil, and China.
Learn more about Unither Pharmaceuticals: www.unither-pharma.com