Your Innovation &
Development partner
Our know-how
Pharmaceutical development
- Formulation
- Pre-clinical
- Clinical
Small batches manufacture
- Clinical
- Commercial
- Consumer/ market tests

Analytical services
Ad hoc and/or part of a pharmaceutical development

Regulatory support
Development strategy & compiling IMPD
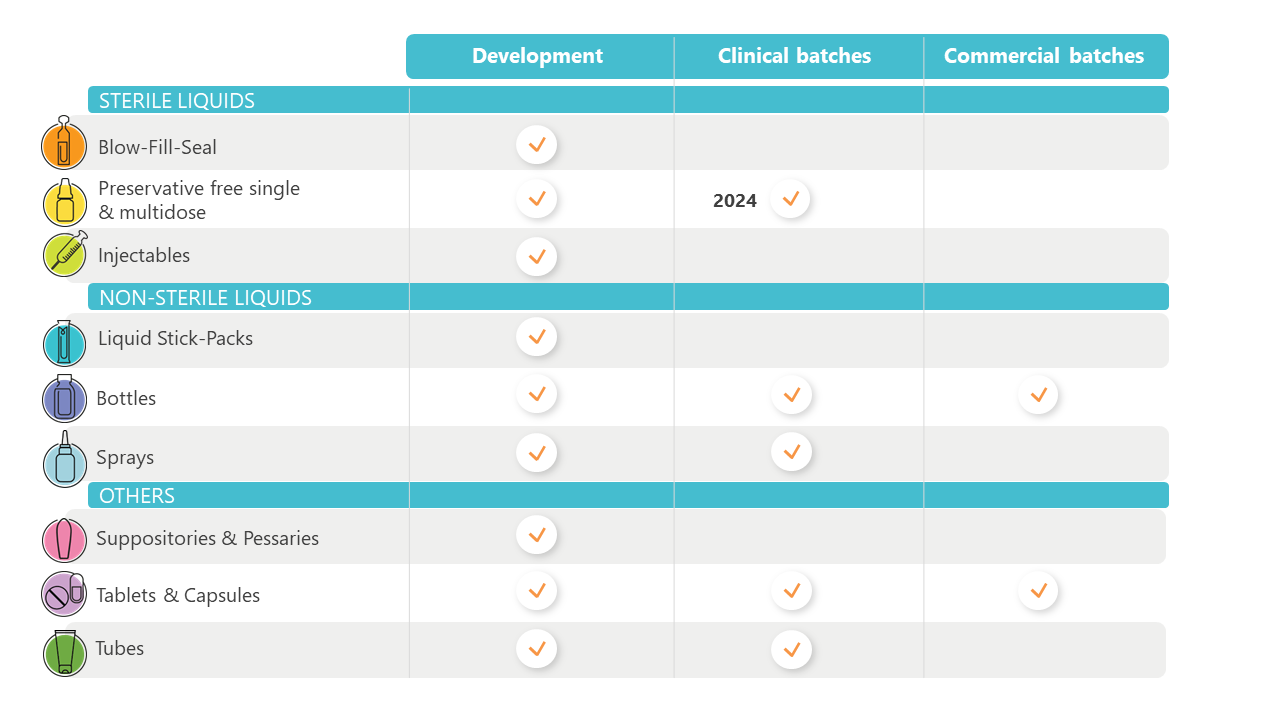
Product forms
(sterile / non sterile)



Solids
Powders, granules, tablets, capsules
Liquids
Solutions, suspensions, syrups, (nano)emulsions
Semi-solids
Gels, creams, ointments, suppositories, pessaries
Our technologies
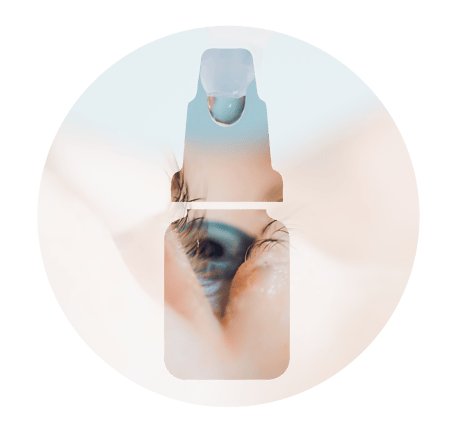
Development of ophthalmic products
NCE, repositioned molecules, hybrids/generics + etc.
Solution, suspension, gel, emulsion formulation and analytical development
.png?width=445&name=MicrosoftTeams-image%20(64).png)
Orphan drugs & Rare diseases
Development / production
Pharmaceutical development
One-stop shop approach for a faster time to market



Analytical services
- Analytical development
- Pharmaceutical development support
- Analytical reverse engineering (formulation support)
- Validation, co-validation, transfer
- Cleaning verification
- Quality control
- ICH / VICH stability studies and preliminary stability studies

.png?width=1200&length=1200&name=uniter%20(1500%20%C3%97%201080%20px).png)



Production services
Manufacturing expertise services
- Manufacturing process development and optimization
- Life cycle management such as clean label/reformulation, new packaging testing
- Pilot and technical batches
- Scale-up & industrial transfer
- Trouble shooting: new regulation compliance, stability remediation, manufacturing process improvement
Product supply
Development area
- Prototypes
- Pre-clinical supplies
GMP batches manufacturing
-
Clinical batches manufacturing (& placebo): Phase I to III
-
Small commercial batches (niche) manufacturing
-
Consumer/market tests
Regulatory support
Development strategy
Compiling IMPD
Pre and post-marketing: clinical study authorization requests (IND, IMPD), registration files (NDA, CTD, safety report, etc.)

Any project? Development or Analytical support?
Contact us!
Fill out this form and we will reach out to you to propose you the best support solutions. Contact us!